Class
#27 ORGANIC CHEMISTRY PART III
Historical Insight Revisited
Formal (Wood) Alcohol => Formaldehyde => Formic Acid
Grain Alcohol => Acetaldehyde => Acetic Acid
Oxidation of Aldehydes produces Carboxylic
Acids
C-C-C-C=O
+ Oxygen => C-C-C-C=O
(R-COOH)
\ OH
Butanal
Butanoic Acid
Alkyl
Substitution of Ammonia produce amines
C
|
C-C-C-NH2
C-C-C-NH-C
C-N-C
Primary
Secondary
Tertiary
Amine
Amine
Amine
(R-NH2)
(R-NH-R)
(R-NR-R)
propylamine
methylpropylamine
trimethylamine
Organic Neutralizations:
Carboxylic Acid + Alcohol => Ester
Carboxylic Acid + Amine => Amide
C-C-C-C=O + C-C-OH
=> C-C-C-C=O
\
OH
\O-C-C
Butanoic
Acid
Ethanol
Ethylbutanate
C-C-C-C=O
+ C-C-NH2
=> C-C-C-C=O
\
OH
\NH2
Butanoic
Acid Ethylamine
butanamide
Important in chemistry of life would be amino acids uses to make
protein.
Cyclic Componds
Alkanes
and Alkenes
Cyclopropane
Cyclopentane
C
C
/
\
/ \
C _
C
C C
| |
C _ C
Cyclohexene
Cyclobutene
C
C = C
/
\
| |
C
C
C _ C
| |
C C
\
//
C
(Cyclohextriene)
Benzene => Phenyl
Group (aryl-) (Aromatic Compounds)

Benzene Functional Groups
Toluene
Phenol
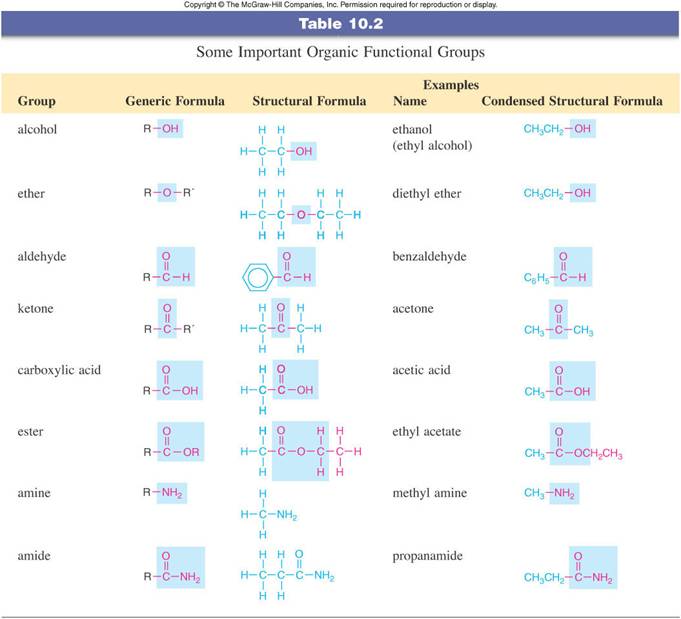
REVIEW OF ORGANIC
FUNCTIONAL GROUPS